EVALUATION OF A METHOD FOR THE DESTRUCTION OF EXPIRED SODIUM METAL ON A LABORATORY SCALE
Keywords:
ethanol; dangerous waste; metallic sodiumAbstract
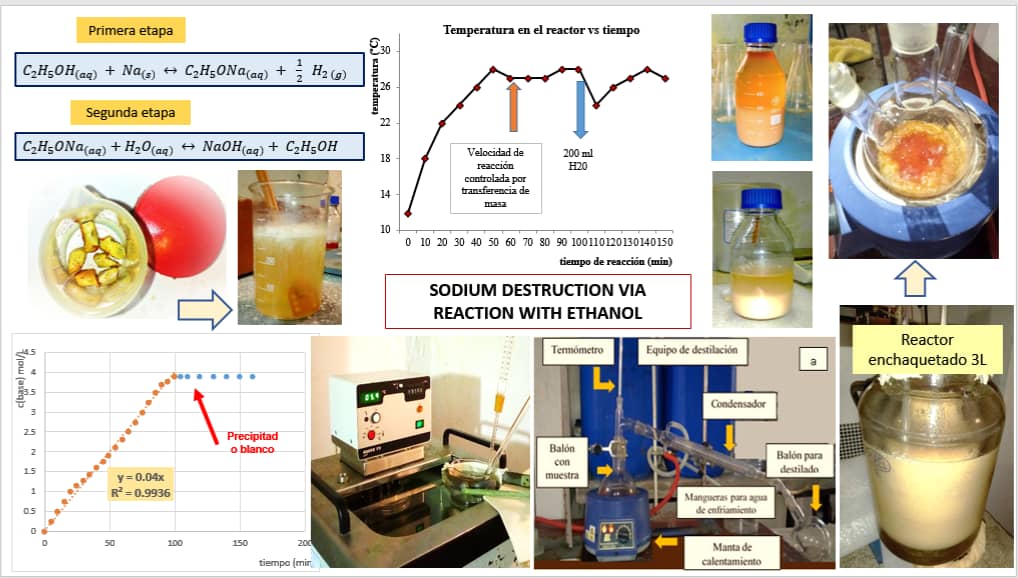
In the present work was to asses, on a laboratory scale, a method for the destruction of expired
metallic sodium under safe conditions. For this, its reaction with ethanol was studied, which
occurs in two stages. The variation of the temperature of the reaction system as a function of the
sodium dosing rate, the mass ratio of technical ethanol/sodium depending on the weight percent
of the ethanol used, the need for water in the second stage, and the stirring speed were
experimentally investigated. From these tests, the parameters were obtained, to safely carry out
the destruction of this metal. Finally, an elemental characterization of the waste formed was
carried out, proposing several alternatives for its management, highlighting that more than 98 %
of the ethanol used in the first stage is recovered by distillation, guaranteeing its reuse
References
ROGALA, M.; WESIERSKI, T. “Determination of
assumptions for neutralization techniques and
protection of metallic sodium residues in rescue
operation conditions”. Zeszyty Naukowe, SGSP. 2023,
(1): pp. 175-194.
https://doi.org/10.5604/01.3001.0054.1457
DAVID, L. et al. “A small scale experiment and a
simplified model to investigate the runaway of
sodium-water reaction”. Int. J. Heat and Mass
Transfer, 2019, 144, 118542. DOI:
https://doi.org/10.1016/j.ijheatmasstransfer.2019.1185
(hal-02095552).
WHITAKER, K. S.; WHITAKER, D. T. "Sodium
Ethoxide" in: Encyclopedia of Reagents for Organic
Synthesis. 1st Edition. New York. Wiley. 2001. Print
ISBN: 9780471936237, online
ISBN: 9780470842898,
DOI: https://doi.org/10.1002/047084289X.rs070
CHEMICAL BOOK. Chemical Safety Data Sheet
MSDS / SDS. Sodium and Sodium ethoxide. Last
review: January 2025.
https://www.chemicalbook.com/ProductIndex_EN.aspx
Hazardous Substances Data Bank (HSDB). Last
review: 18.01.2025 website:
https://toxnet.nlm.nih.gov/newtoxnet/hsdb.htm
IPCS. The International Chemical Safety Cards,
(last review): 04.11.2024. website:
http://www.ilo.org/dyn/icsc/showcard.home
LAKSHMANAN, A. R. et al. “A novel method of
non-violent dissolution of sodium metal in a
concentrated aqueous solution of Epsom salt”.
Journal of Solid State Chemistry, 2004, 177(10): pp.
-3468, ISSN 0022-4596,
https://doi.org/10.1016/j.jssc.2004.05.030
BRAULT, A. et al. “Destruction of contaminated
metallic sodium wastes by reaction on alcohol and
hydrolysis”. CEA, Centre d'Etudes Nucleaires de
Fontenay-aux-Roses, 92 (France). Dept. de
Protection. 1977, pp. 60-80. OIEA. Report number:
CEA-N--1955. Actualizado y revisado: diciembre
HERRMANN, S. et al. “Controlled Conversion of
Sodium Metal from Nuclear Systems to Sodium
Chloride”, JNFCWT, 2021, 19(2). 233-241. eISSN 2288-5471 / pISSN 1738-1894.
https://doi.org/10.7733/jnfcwt.2021.19.2.233
IAEA-TECDOC-1524. Radioactive sodium waste
treatment and conditioning. IAEA, Vienna, 2007. pp.
-44. ISBN 92-0-116006-2. ISSN 1011-4289.
Printed by the IAEA in Austria, January 2007.
ROESKY, H. W. “A Facile and Environmentally
Friendly Disposal of Sodium and Potassium with
Water”. Inorg. Chem. 2001, 40(26): pp. 6855-6856.
DOI: https://doi.org/10.1021/ic010594m
CHANDRAN, K. et al. “Synthesis and
characterization of sodium alkoxides”. Bull Mater
Sci., 2006, 29, p. 173-179.
https://doi.org/10.1007/BF02704612
CHANDRAN, K. et al. “Standard molar
enthalpies of formation of sodium alkoxides”, J.
Chem. Thermodynamics, 2007, 39(3): pp 449-454.
https://doi.org/10.1016/j.jct.2006.07.024
KESSLER, V.G. “Metal alkoxides as models for
metal oxides-the concept revisited”. J. Sol-Gel Sci
Technol., 2024, 112: pp. 502-511.
https://doi.org/10.1007/s10971-024-06548-w
BESKE, M.; TAPMEYER, L; SCHMIDT, M.
“Crystal structure of sodium ethoxide (C2H5ONa),
unravelled after 180 years”, Chemical
communications, 2020, 56(24): pp. 3520-3523.
https://doi.org/10.1039/C9CC08907A
GHARGE, M. S.; KAMBLE, V. M. “Synthesis
and characterization of sodium alkoxide as organic
reagent”. Journal of Emerging Technologies and
Innovative Research (JETIR). March 2021, 8(3): pp.
-2575. ISSN-2349-5162. eISSN: 2349-5162.
BESKE, M. et al. “Disordered sodium alkoxides
from powder data: crystal structures of sodium
ethoxide, propoxide, butoxide and pentoxide, and
some of their solvates”. Structural Science, Crystal
Engineering and Materials. 2021, 77(Part 1): pp. 68-
ISSN: 2052-5206.
https://doi.org/10.1107/S205252062001584X
RANDRIANA, N. R.;
RANDRIANOMENJANAHARY, A. M;
RABEHARITSARA, A. T. “Sodium Ethoxide
Concentrated Solution Synthesis at Ambient
Temperature Using Sodium Hydroxide and Ethanol90 in Excess”. World Journal of Applied Chemistry,
, 6(1): pp. 6-11. ISSN: 2637-5982.
https://doi.org/10.11648/j.wjac.20210601.12
SHANDONG HUIHAI PHARMACEUTICAL
& CHEMICAL Co. Ltd. “Preparation of metal
alcoholates by converting hydroxy groups to Ometal groups” Denomination of invention: A
preparation method of sodium ethoxide. Patent:
[C07C29], R. P. China, Registration number:
Y2021980013568, 2021-11-30.
DAVID, L.; MILANOVIC, M.; HERVE, P. et al.
“Spectroscopic, pressure and temperature
measurements of the reactant mixing process in
sodium-water reaction”. Nuclear Eng. and Design,
364, 110638.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Yamell Jiménez-Prieto, Guillermo Esperanza-Pérez, Surey Ramírez-González, Javier Martin-Santin, Juan A. Ribalta-Quesada

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
This journal provides immediate open access to its content, based on the principle that offering the public free access to research helps a greater global exchange of knowledge. Each author is responsible for the content of each of their articles.






















