PREPARATION IN AQUEOUS MEDIUM OF AMORPHOUS TiO2 COATINGS ON LNMO PARTICLES
Keywords:
recubrimiento superficial amorfo; LNMO; TiO2; baterías de ion litio.Abstract
Translator
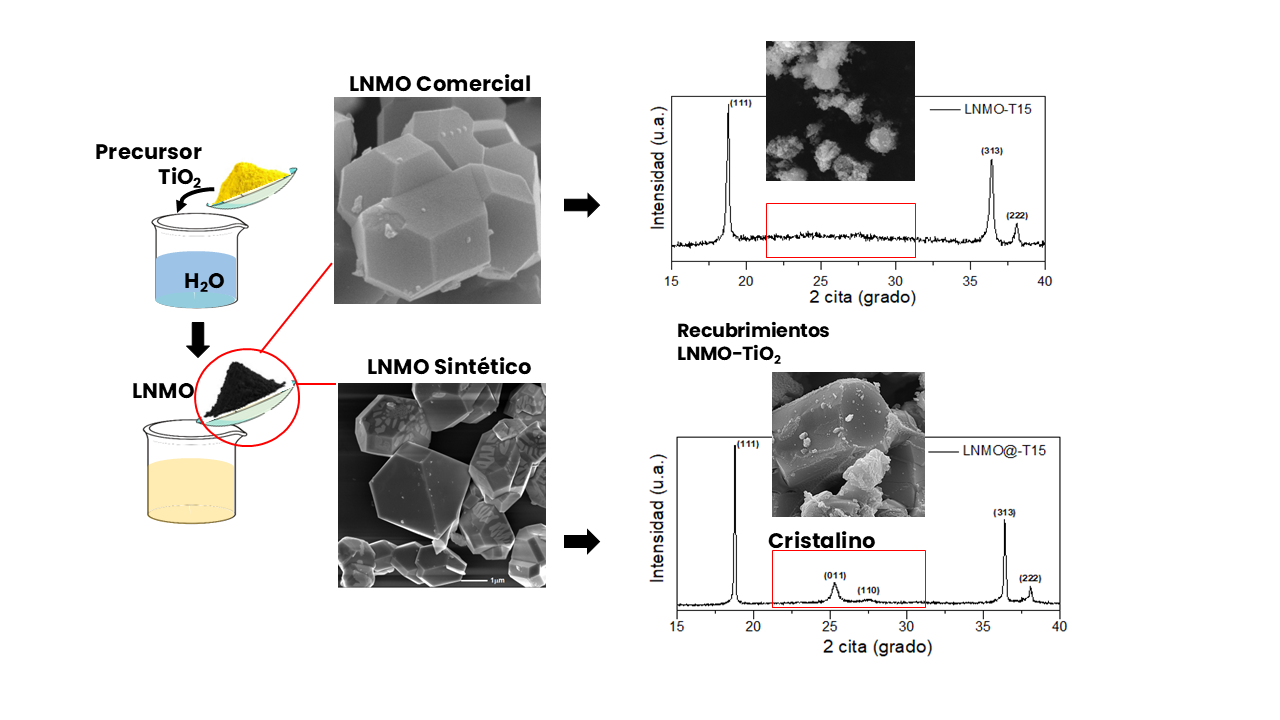
LiNi0.5Mn1.5O4 (LNMO) is a promising high-voltage cathode material; however, its capacity is degraded by reactions with the electrolyte. Undesired reactions between the cathode and electrolyte in Li-ion batteries can be avoided by surface-coating the active cathode material. We used the citrate method in an aqueous medium to coat the commercial LNMO particles with amorphous TiO2. Three coatings were obtained to evaluate composition, morphology, structure thermal stability and electronic conductivity. Several characterization techniques were used: X-ray fluorescence, scanning electron microscopy, X-ray diffraction, Raman spectroscopy, and attenuated total reflection. The electronic conductivity was measured using the Kelvin Method. Among the prepared coatings, the best was the LNMO-T3, given that the amorphous TiO2 phase homogeneously covered the LNMO core particles with a morphology that resembled the physical core-shell type coating, a higher thermal stability, and electronic conductivity similar to that of the LNMO core material.
References
KUENZEL, M. et al. “Crystal engineering of
TMPOx-coated LiNi0. 5Mn1. 5O4 cathodes for highperformance lithium-ion batteries”. Materials Today.
, 39, 127-136.
https://doi.org/10.1016/j.mattod.2020.04.003
QURESHI, Z. A. et al. “Impact of coatings on the
electrochemical performance of LiNi0.5Mn1.5O4
cathode materials: A focused review”. Ceramics
International. 2022, 48(6), 7374-7392.
https://doi.org/10.1016/j.ceramint.2021.12.118
WU, P.; ZHANG, Y. “Enhanced Electrochemical
Performance of Zr4+ and Co3+ doped LiNi0.65Mn0.35O2
Cathode Material for Lithium Ion Batteries”. International Journal of Electrochemical Science.
, 17(6), 220646.
https://doi.org/10.20964/2022.06.48
CHEN, T. et al. “The CeF4-coated spinel LiNi0.5Mn1.5O4
with improved electrochemical performance for 5 V
lithium-ion batteries”. Journal of Materials Science:
Materials in Electronics. 2022, 33(15), 11712-11724.
https://doi.org/10.1007/s10854-022-08137-5
PILLAI, A. M. et al. “Surface engineering of
Li1.5Ni0.25Mn0.75O2.5 cathode material using TiO2
nanoparticles: An approach to improve
electrochemical performance and thermal stability”.
Journal of Alloys and Compounds. 2024 976, 173064.
https://doi.org/10.1016/j.jallcom.2023.173064
DAI, S. et al. “Enhanced high-rate cycling
performance of LiMn2O4 cathode materials by coating
nano-TiO2”. International Journal of Materials
Research. 2023, 114(1), 7-15.
https://doi.org/10.1515/ijmr-2022-0070
WAGEMAKER, M. et al. “Two phase morphology
limits lithium diffusion in TiO(2)(anatase): a (7)Li
MAS NMR study”. Journal of the American
Chemical Society. 2001, 123(46), 11454-11461.
https://doi.org/10.1021/ja0161148
MOITZHEIM, S.; DE GENDT, S.; VEREECKEN,
P. M. “Investigation of the Li-Ion Insertion
Mechanism for Amorphous and Anatase TiO2 ThinFilms”. Journal of The Electrochemical Society. 2019,
(2), A1. https://doi.org/10.1149/2.1091816jes
YILDIRIM, H.; GREELEY, J.;
SANKARANARAYANAN, S. K. “Effect of
concentration on the energetics and dynamics of Li
ion transport in anatase and amorphous TiO2”. The
Journal of Physical Chemistry C. 2011, 115(31),
-15673.
https://pubs.acs.org/doi/10.1021/jp202514j
HAO, X.; BARTLETT, B. M. “Improving the
Electrochemical Stability of the High-Voltage Li-Ion
Battery Cathode LiNi0.5Mn1.5O4 by Titanate-Based
Surface Modification”. Journal of The
Electrochemical Society . 2013, 160(5), A3162.
https://doi.org/10.1149/2.025305jes
SONG, Y. W. et al. “Surface Modification of
High Voltage Spinel LiNi0.5Mn1.5O4 Cathode Material
Manufactured via Co-precipitation”. Journal of The
Electrochemical Society . 2024, 171(5), 050558.
https://doi.org/10.1149/1945-7111/ad4e6f
FEINER, A. S.; MCEVOY, A. J.. “The Nernst
Equation”. Journal of Chemical Education. 1994,
(6), 493. https://doi.org/10.1021/ed071p493
DAKANALI, M. et al. “A New Dinuclear
Ti(IV)−Peroxo−Citrate Complex from Aqueous
Solutions. Synthetic, Structural, and Spectroscopic
Studies in Relevance to Aqueous
Titanium(IV)−Peroxo−Citrate Speciation”. Inorganic
Chemistry. 2003, 42(15), 4632-4639.
https://doi.org/10.1021/ic0343051
SAVINKINA, E. V. ET AL. “Introduction of
peroxo groups into titania: Preparation,
characterization and properties of the new peroxocontaining phase”. CrystEngComm. 2015, 17(37),
-7123. https://doi.org/10.1039/C5CE01090J
WANG, L. et al. “A comparative study of Fd-3m
and P4332 “LiNi0.5Mn1.5O4”. Solid State Ionics. 2011,
(1), 32-38.
https://doi.org/10.1016/j.ssi.2011.04.007
NAKAMOTO, K. Infrared and Raman Spectra of
Inorganic and Coordination Compounds, Part A:
Theory and Applications in Inorganic Chemistry. 6ta
Edición. New Jersey: Wiley, 2008. ISBN: 978-0-471-
-2
BICHARA, L. C. et al. “Vibrational Study and
Force Field of the Citric Acid Dimer Based on the
SQM Methodology”. Advances in Physical
Chemistry. 2011, 2011 347072.
https://doi.org/10.1155/2011/347072
WU, H.; CHAN, M.; CHAN, C. “FTIR
Characterization of Polymorphic Transformation of
Ammonium Nitrate”. Aerosol Science and
Technology. 2007, 41, 581-588.
https://doi.org/10.1080/02786820701272038
BANERJEE, S.; KUMAR, A.; DEVI, P.
“Preparation of nanoparticles of oxides by the citratenitrate process”. Journal of Thermal Analysis and
Calorimetry. 2011, 104, 859-867.
https://doi.org/10.1007/s10973-011-1525-6
ZHANG, B.; WANG, Z.; & GUO, H.. “Effect of
annealing treatment on electrochemical property of
LiNi0.5Mn1.5O4 spinel”. Transactions of Nonferrous
Metals Society of China. 2007, 17(2), 287-290.
https://doi.org/10.1016/S1003-6326(07)60086-7
ZHONG, Q. et al. “Synthesis and
Electrochemistry of LiNixMn2−xO4”. Journal of The
Electrochemical Society. 1997, 144(1), 205.
https://doi.org/10.1149/1.1837386
ADAMCZYK, A.; DŁUGOŃ, E. “The FTIR
studies of gels and thin films of Al2O3-TiO2 and
Al2O3-TiO2-SiO2 systems”. Spectrochimica Acta Part
A: Molecular and Biomolecular Spectroscopy. 2012,
, 11-17. https://doi.org/10.1016/j.saa.2011.12.018
OKUDUR, F. U. et al. “Ti surface doping of
LiNi0.5Mn1.5O4−δ positive electrodes for lithium ion
batteries”. RSC Advances. 2018, 8, 7287-7300.
https://doi.org/10.1039/C7RA12932G
BHATIA, A. et al. “Detailed redox mechanism
and self-discharge diagnostic of 4.9 V LiMn1.5Ni0.5O4
spinel cathode revealed by Raman spectroscopy”.
Journal of Materials Chemistry A. 2021, 9, (13496-
. https://doi.org/10.1039/D1TA00989C
HIROI, Z. “Inorganic structural chemistry of
titanium dioxide polymorphs”. Inorganic Chemistry.
, 61(22), 8393-8401.
https://pubs.acs.org/doi/10.1021/acs.inorgchem.2c009
CHEN, Z. et al. “Role of surface coating on
cathode materials for lithium-ion batteries”. Journal
of Materials Chemistry. 2010, 20(36), 7606-7612.
https://doi.org/10.1039/C0JM00154F
MUNIR, S. et al. “Effect of carrier concentration
on the optical band gap of TiO2 nanoparticles”.
Materials & Design. 2016, 92, 64-72.
https://doi.org/10.1016/j.matdes.2015.12.022
DENG, J. et al. “Improving the fast discharge
performance of high-voltage LiNi0.5Mn1.5O4 spinel by
Cu2+, Al3+, Ti4+ tri-doping”. Journal of Alloys and
Compounds. 2016, 677, 18-26.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Ana Laura Díaz-Perera, Carlos Ricardo Milián-Pila, Manuel Ávila-Santos, Yodalgis Mosqueda-Laffita, Eduardo Lázaro Pérez-Cappe

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
This journal provides immediate open access to its content, based on the principle that offering the public free access to research helps a greater global exchange of knowledge. Each author is responsible for the content of each of their articles.






















